beware of such nonsense in the emergency room at north shore univerdity hospital.
hold your loved one's hand, speak to them etc but do not give elderly , 94 years old patients Hslfol
Generic Name: haloperidol lactate
Dosage Form: injection
Dosage Form: injection
(For Immediate Release)
WARNING
Increased Mortality in Elderly Patients with Dementia-Related Psychosis
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Analyses of seventeen placebo-controlled trials (modal duration of 10 weeks), largely in patients taking atypical antipsychotic drugs, revealed a risk of death in drug-treated patients of between 1.6 to 1.7 times the risk of death in placebo-treated patients. Over the course of a typical 10-week controlled trial, the rate of death in drug-treated patients was about 4.5%, compared to a rate of about 2.6% in the placebo group. Although the causes of death were varied, most of the deaths appeared to be either cardiovascular (e.g., heart failure, sudden death) or infectious (e.g., pneumonia) in nature. Observational studies suggest that, similar to atypical antipsychotic drugs, treatment with conventional antipsychotic drugs may increase mortality. The extent to which the findings of increased mortality in observational studies may be attributed to the antipsychotic drug as opposed to some characteristic(s) of the patients is not clear. Haldol Injection is not approved for the treatment of patients with dementia-related psychosis (see WARNINGS).
Haldol Description
Haloperidol is the first of the butyrophenone series of major antipsychotics. The chemical designation is 4-[4-(p-chlorophenyl)-4-hydroxypiperidino]-4'-fluorobutyrophenone and it has the following structural formula:
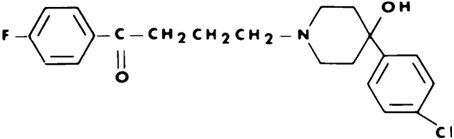
Haldol (haloperidol) is available as a sterile parenteral form for intramuscular injection. The injection provides 5 mg haloperidol (as the lactate) and lactic acid for pH adjustment between 3.0 – 3.6.
ACTIONS
The precise mechanism of action has not been clearly established.
INDICATIONS
Haldol (haloperidol) is indicated for use in the treatment of schizophrenia.
Haldol is indicated for the control of tics and vocal utterances of Tourette's Disorder.
Contraindications
Haldol (haloperidol) is contraindicated in severe toxic central nervous system depression or comatose states from any cause and in individuals who are hypersensitive to this drug or have Parkinson's disease.
Warnings
Increased Mortality in Elderly Patients with Dementia-Related Psychosis
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Haldol Injection is not approved for the treatment of patients with dementia-related psychosis (see BOXED WARNING).
Cardiovascular Effects
Cases of sudden death, QT-prolongation, and Torsades de Pointes have been reported in patients receiving Haldol. Higher than recommended doses of any formulation and intravenous administration of Haldol appear to be associated with a higher risk of QT-prolongation and Torsades de Pointes. Although cases have been reported even in the absence of predisposing factors, particular caution is advised in treating patients with other QT-prolonging conditions (including electrolyte imbalance [particularly hypokalemia and hypomagnesemia], drugs known to prolong QT, underlying cardiac abnormalities, hypothyroidism, and familial long QT-syndrome). Haldol INJECTION IS NOT APPROVED FOR INTRAVENOUS ADMINISTRATION. If Haldol is administered intravenously, the ECG should be monitored for QT prolongation and arrhythmias.
Tardive Dyskinesia
A syndrome consisting of potentially irreversible, involuntary, dyskinetic movements may develop in patients treated with antipsychotic drugs. Although the prevalence of the syndrome appears to be highest among the elderly, especially elderly women, it is impossible to rely upon prevalence estimates to predict, at the inception of antipsychotic treatment, which patients are likely to develop the syndrome. Whether antipsychotic drug products differ in their potential to cause tardive dyskinesia is unknown.
Both the risk of developing tardive dyskinesia and the likelihood that it will become irreversible are believed to increase as the duration of treatment and the total cumulative dose of antipsychotic drugs administered to the patient increase. However, the syndrome can develop, although much less commonly, after relatively brief treatment periods at low doses.
There is no known treatment for established cases of tardive dyskinesia, although the syndrome may remit, partially or completely, if antipsychotic treatment is withdrawn. Antipsychotic treatment, itself, however, may suppress (or partially suppress) the signs and symptoms of the syndrome and thereby may possibly mask the underlying process. The effect that symptomatic suppression has upon the long-term course of the syndrome is unknown.

No comments:
Post a Comment